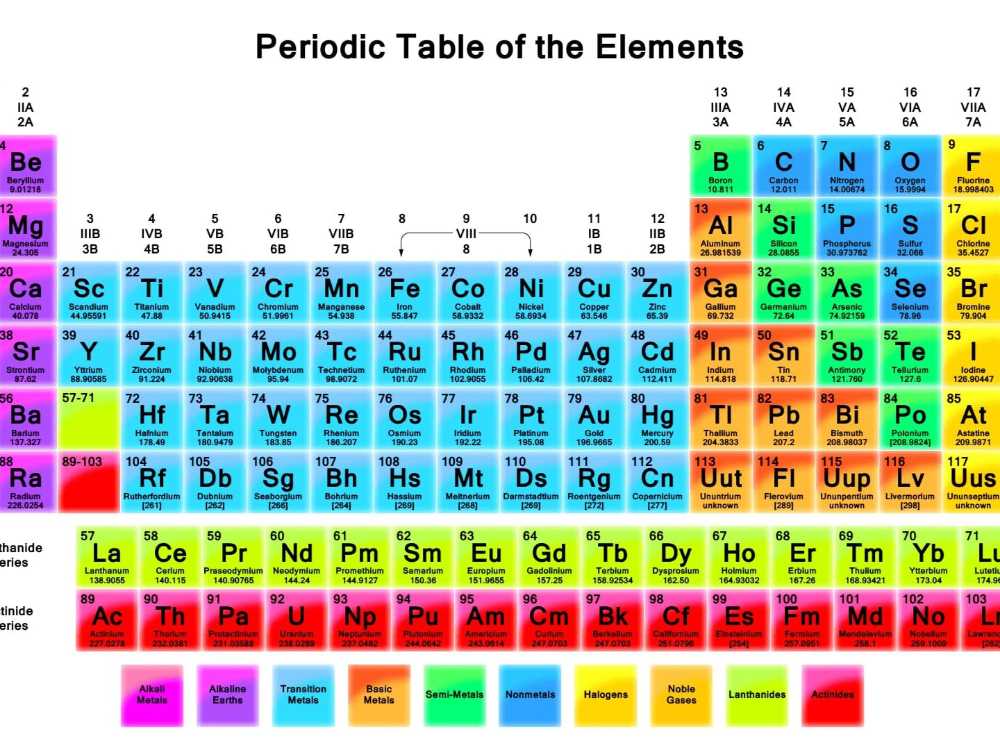
When it comes to chemistry, chemical symbols play a vital role in representing elements and their properties. One such symbol, 1s22s2, holds significance in terms of electron configuration.
Electron configuration refers to the arrangement of electrons in an atom’s orbitals. In the case of 1s22s2, the symbol represents the electron configuration for an element with two energy levels or shells. The first shell, labeled 1s, contains two electrons, while the second shell, labeled 2s, also contains two electrons.
Based on the periodic table, we can deduce the element to which this electron configuration belongs. The 1s22s2 configuration corresponds to the element beryllium. Beryllium (Be) is a lightweight, alkaline earth metal that is commonly used in various industries due to its high strength and low density.
Understanding and interpreting chemical symbols, such as 1s22s2, allows scientists and chemists to identify elements and predict their behavior in chemical reactions. These symbols serve as a universal language in the world of chemistry, facilitating communication and research in this field.
s22s2 Express Your Answer as a Chemical Symbol
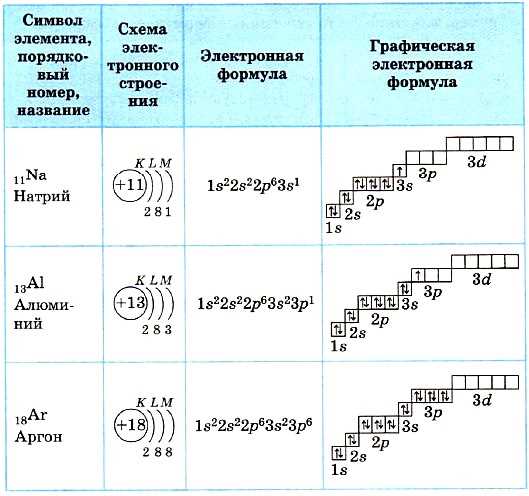
In chemistry, chemical symbols are used to represent elements and compounds. These symbols are shorthand notations that help to convey information about the composition of a substance. One example of a chemical symbol is s22s2.
The chemical symbol s22s2 represents an unknown element or compound. Without further information, it is difficult to determine the exact identity of this substance. However, by analyzing the symbol, we can make some assumptions. The presence of two s’s suggests that sulfur may be involved, as the chemical symbol for sulfur is S. The number 22 could indicate the atomic number or mass number of the element.
To fully express the chemical symbol s22s2, additional context or information would be needed. This could include the atomic number, mass number, or the positions of the elements in a compound. Without this information, the symbol remains ambiguous.
Chemical symbols play a crucial role in chemistry, allowing scientists to communicate complex information concisely. They are often used in formulas, equations, and chemical equations to represent elements and compounds. By knowing the chemical symbols for different elements, scientists can quickly identify substances and understand their properties and behavior.
Understanding the Chemical Symbol Notation
The chemical symbol notation is a way of representing elements in chemistry. Each element is assigned a unique symbol, typically consisting of one or two letters, which is used to identify and describe the element. This notation is essential in communicating and studying chemical reactions and compounds.
Atomic Number: The chemical symbol notation is closely related to an element’s atomic number. The atomic number represents the number of protons in an atom’s nucleus. For example, the chemical symbol for hydrogen is H, which corresponds to its atomic number of 1.
Mendeleev’s Periodic Table: The chemical symbol notation was popularized by Dmitri Mendeleev, who created the periodic table of elements. The periodic table organizes elements based on their chemical properties and atomic number. Each element is represented by its chemical symbol and is arranged in order of increasing atomic number.
One or Two Letters: Most of the chemical symbols consist of one or two letters. The first letter is always capitalized, while the second letter, if present, is lowercase. For example, carbon is represented by the symbol C, while nitrogen is represented by the symbol N.
Exceptions: Some chemical symbols are derived from their Latin or Greek names. For instance, iron is represented by Fe, which comes from the Latin word “ferrum.” Similarly, gold is represented by Au, from the Latin word “aurum.”
International Convention: The chemical symbol notation follows an international convention established by the International Union of Pure and Applied Chemistry (IUPAC). This convention ensures consistency and uniformity in the representation of elements across different languages and regions.
Chemical Formulas: The chemical symbol notation is also used to write chemical formulas, which represent the composition of compounds. In compound formulas, the chemical symbols of the elements are combined with subscripts to indicate the number of atoms of each element present.
In conclusion, understanding the chemical symbol notation is fundamental in chemistry. It allows scientists, students, and researchers to communicate and identify elements accurately. Through the chemical symbol notation, the vast diversity of elements and their compounds can be organized and studied systematically.
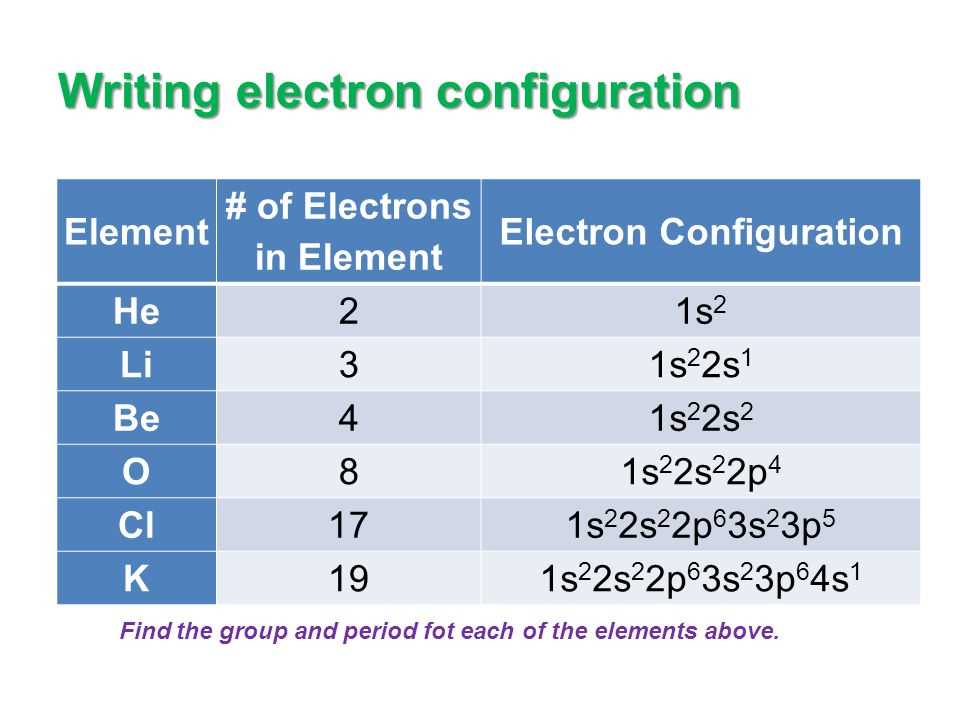
Electron Configuration of Elements

The electron configuration of an element refers to the arrangement of electrons in its atomic orbitals. It provides important information about the chemical behavior and properties of an element. The electron configuration can be represented using a series of numbers, letters, and superscripts.
The electron configuration is based on the Aufbau principle, which states that electrons fill atomic orbitals in order of increasing energy. Each electron occupies the lowest-energy orbital available before filling higher-energy orbitals. The electron configuration of an element can be determined by following the rules of the Aufbau principle.
In general, the electron configuration is represented using the following notation: {1s^2 2s^2 2p^6 3s^2 3p^6 …}, where the numbers and letters represent the different orbitals and superscripts indicate the number of electrons in each orbital. For example, the electron configuration of oxygen is {1s^2 2s^2 2p^4}, indicating that it has two electrons in the 1s orbital, two electrons in the 2s orbital, and four electrons in the 2p orbital.
The electron configuration of an element can also be represented using a shorthand notation called the noble gas configuration. This notation uses the symbol of the noble gas that comes before the element in the periodic table and represents the full electron configuration of the noble gas in brackets. For example, the electron configuration of oxygen can be represented as [He] 2s^2 2p^4, indicating that it has the same electron configuration as helium up to the 2s orbital.
Understanding the electron configuration of elements is essential in predicting their chemical reactivity and forming chemical compounds. It helps in explaining trends in the periodic table and the formation of chemical bonds. Additionally, the electron configuration provides insights into the stability and energy levels of an atom.
Grouping Elements by Chemical Symbols
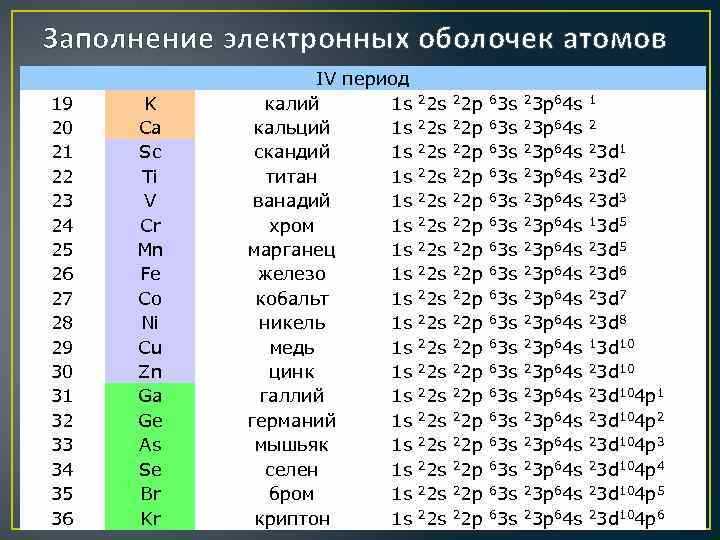
Chemical symbols are used to represent elements in the periodic table. They are composed of one or two letters and provide a concise way to identify and refer to specific elements. By grouping elements based on their chemical symbols, we can better understand their properties and relationships.
One way to group elements is by their first letter in the chemical symbol. For example, all elements with the symbol “H” belong to the group of hydrogen. This includes hydrogen itself, as well as other elements like helium (He) and hafnium (Hf). By organizing elements in this way, we can easily identify common characteristics and trends within a group.
Another way to group elements is by their second letter in the chemical symbol. For example, all elements with the symbol “O” as their second letter belong to the oxygen group. This includes elements like boron (B), carbon (C), and fluorine (F). By grouping elements in this manner, we can explore similarities and differences in their chemical behavior and properties.
The use of chemical symbols allows scientists and researchers to communicate and study elements more efficiently. By grouping elements based on their symbols, we can identify patterns, make predictions, and discover new relationships between elements. This organized approach helps to streamline scientific research and deepen our understanding of the building blocks of matter.
How to Express an Answer Using Chemical Symbols
When solving chemistry problems, it is essential to express answers using chemical symbols. Chemical symbols are shorthand representations of elements, which allow for concise and precise communication in the field of chemistry. Here are some tips on how to express an answer using chemical symbols.
1. Identify the elements: Before expressing an answer using chemical symbols, it is crucial to identify the elements involved in the problem. This can be done by analyzing the given information, such as the compound’s formula or the reactants and products in a chemical reaction.
2. Write the chemical formula: Once the elements are identified, it is time to write their respective chemical symbols. Chemical symbols are usually one or two letters long and are derived from the element’s name, often in Latin or German. For example, the chemical symbol for oxygen is “O,” and the symbol for sodium is “Na.”
3. Use subscripts: In many cases, chemical formulas require the use of subscripts to indicate the number of atoms of each element in a compound. Subscripts are written as small numbers below and to the right of the chemical symbol. For example, the chemical formula for water is “H2O,” indicating two hydrogen atoms and one oxygen atom.
4. Consider charges: In some cases, elements may have different charges, known as oxidation states, depending on the specific situation. When expressing an answer involving charged elements, it is essential to indicate their charges using superscripts. Superscripts are written as small numbers above and to the right of the chemical symbol. For example, the chemical formula for sodium chloride, a compound formed by sodium (Na) and chlorine (Cl), is “Na+Cl–,” indicating the one positive charge on sodium and the one negative charge on chlorine.
5. Check for accuracy: Finally, before submitting your answer, it is crucial to double-check the accuracy of the chemical symbols and their arrangement. Mistakes in expressing chemical symbols can lead to incorrect answers or confusion in the field of chemistry.
By following these steps, you can effectively express your answer using chemical symbols, ensuring clear and accurate communication in the field of chemistry.
Examples of Expressing Answers as Chemical Symbols
Chemical symbols are shorthand notations used to represent elements and compounds in chemistry. Expressing answers as chemical symbols is a common practice in the field, as it allows for concise and precise communication. Here are a few examples of how chemical symbols can be used to express answers in various contexts.
1. Naming Elements: Chemical symbols are used to represent elements, which are the building blocks of matter. For example, the chemical symbol for oxygen is O, and for hydrogen is H. These symbols are used to express the presence of these elements in chemical equations or formulas.
- Oxygen: O
- Hydrogen: H
2. Writing Chemical Formulas: Chemical formulas are used to represent compounds, which are substances made up of two or more elements. For example, the chemical formula for water is H2O, where H represents hydrogen and O represents oxygen. This formula expresses the composition of water in terms of its constituent elements.
- Water: H2O
3. Balancing Chemical Equations: Chemical equations are used to represent chemical reactions. Balancing equations involves ensuring that the number of atoms of each element is equal on both sides of the equation. Chemical symbols are used to express the presence and quantities of elements in the reaction.
For example, the combustion of methane can be represented by the following balanced equation:
CH4 + 2O2 → CO2 + 2H2O
Here, CH4 represents methane, O2 represents oxygen, CO2 represents carbon dioxide, and H2O represents water.
Overall, expressing answers as chemical symbols is an important skill in the field of chemistry. It allows for clear and concise communication of the elements, compounds, and reactions involved in various chemical processes.
Benefits of Using Chemical Symbols
Chemical symbols provide a concise and standardized way to represent elements and compounds in the field of chemistry. They are essential for effective communication and understanding between scientists, researchers, and students.
1. Universal Language: Chemical symbols, such as H for hydrogen or O for oxygen, are internationally recognized and understood by chemists worldwide. This universal language allows scientists from different countries to communicate and exchange information, regardless of their native languages.
2. Efficient Communication: Chemical symbols provide a shorthand to represent elements and compounds in a compact form. Instead of writing out the full name of an element or compound, chemists can use symbols, such as Cu for copper or CO2 for carbon dioxide, to convey complex ideas or formulas in a more concise and efficient manner.
3. Clarity and Precision: Chemical symbols have specific meanings and eliminate potential confusion or ambiguity that may arise from using common or arbitrary names for elements and compounds. For example, using the symbol Fe to represent iron ensures that there is no confusion between the metal and a word like “iron” that may have other non-chemical meanings.
4. Standardization: Chemical symbols are part of a standardized system, such as the periodic table, which provides a consistent framework for organizing and understanding elements and compounds. This standardization allows for easier comparison of data and promotes collaboration and accuracy in scientific research.
5. Efficiency in Writing and Documentation: Chemical symbols save time and space in various forms of scientific writing and documentation, such as research papers, lab reports, and chemical equations. Their use allows for more concise and organized representation of chemical information, making it easier to convey complex ideas and formulas.
Overall, the use of chemical symbols is essential for effective communication, efficient representation of chemical information, and standardized understanding of elements and compounds in the field of chemistry. They provide a universal language and ensure precision, clarity, and efficiency in scientific research and education.